With the sharp increase in the number of pet cats in recent years, the demand for cat litter, an emerging environmentally friendly product, is also increasing. There are many types of cat litter products on the market, each with different compositions.
The main ingredient of silica gel cat litter is amorphous SiO2. Silica gel particles have good strength but poor adsorption capacity and are easy to penetrate. Moreover, silica gel particles produce more heat after absorbing water, raising the temperature of the pet cat’s living environment, thus promoting bacterial growth. Pine cat litter is lightweight and provides a high level of pet comfort but is generally inadequate for ammonia removal and is more expensive. Conventional bentonite cat litter, with calcium-based bentonite as the main ingredient, is cheap and absorbent but has a poor caking effect, ammonia removal, and is prone to generating dust, affecting pets’ living comfort.

This study represents an improvement on traditional bentonite cat litter. The addition of the porous material zeolite increased the absorption effect of cat litter on ammonia. Sedation of bentonite can be used as both an adsorbent and a binder to enhance the strength of cat litter particles while reducing dust generation. The new cat litter, with bentonite as the main ingredient, has a low preparation cost, excellent effect, and broad market prospect.
Test Part
Raw Materials
The materials used in the experiment are calcium-based bentonite and oblique hair zeolite purified by the cyclone group. The purified bentonite was dried, ground, and then analyzed for chemical composition and performance indicators. The chemical composition of the raw materials is detailed in Table 1, while the performance indicators of bentonite are described in Table 2.
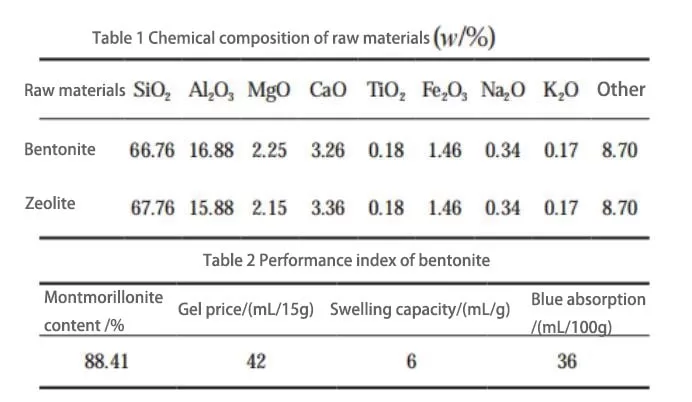
According to the analysis of Table 1 and Table 2, it can be learned that the test used in the bentonite SiO2 and Al2O3 mass ratio of 3.96 is more significant than the theoretical silica-aluminum ratio of 2.36, indicating that the sample contains a small number of impurities. Na2O and K2O’s total content is 0.51%, much smaller than CaO’s 3.26%, indicating that the test sample is a typical calcium-based bentonite. In Table 2, the Montmorillonite content is 88.14%. The zeolite samples contain other metal oxides besides SiO2 and Al2O3 fractions. Zeolite is a porous mineral consisting of silica-oxygen tetrahedra and aluminum-oxygen tetrahedra, which form the basic skeleton. Since the electronic valence of one oxygen atom in the aluminum-oxygen tetrahedra is not neutralized, the aluminum-oxygen tetrahedra carry a one-unit negative charge, and to remain electrically neutral, the zeolite will adsorb other metal cations (Mn+) to counteract its negative charge.
After a long period of geological action, many metal cations are adsorbed, and ion exchange occurs. As a result, the zeolite contains a high level of other metal ions, which are presented as oxides in the physical phase analysis. The mass ratio of SiO2 to Al2O3 in the raw zeolite used is about 4.27, which is between 4 and 8 and belongs to medium silica zeolite.
Experimental Reagents and Experimental Apparatus and Equipment
The chemicals provided include sodium carbonate (Na2CO3), ammonium chloride (NH4Cl), and hydrochloric acid (HCl), all of which are treated with analytical purity. Experimental equipment is also available, including an electronic balance, an electric blast thermostatic oven, an X-ray diffractometer, an assay sample preparation pulverizer, a roller extruder, and a magnetic stirrer of constant temperature.
Test Method
A certain amount of calcium-based bentonite containing impurities was desanded, purified at 105 °C, and then crushed to prepare samples. The semi-dry sodic modification was carried out using Na2CO3 as a sonicating agent under different dosages and roller extrusion. The zeolite-milled samples were sieved and sorted to remove the -100 μm grain size for light-firing tests. The modified samples were mixed with light-fired activated zeolite powder in different ratios with humidity adjustment and extrusion to make short columnar cat litter.
Results and Discussion
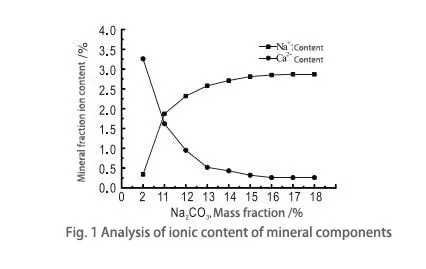
Fig. 1 shows that as the mass fraction of sodium ions in the sodium carbonate solution increases, the calcium ion content of bentonite minerals decreases. In contrast, the sodium ions show a clear increasing trend. This is because the charge equilibrium is disrupted when iron ions replace silicon ions in the tetrahedral structure of montmorillonite or when aluminum ions are replaced by magnesium ions, etc., in the octahedral structure of montmorillonite. The calcium ions adsorbed in the interlayers can only restore the charge equilibrium. Calcium ions have a low bonding energy between layers and are easily replaced by sodium ions or other ions. Therefore, when the mass fraction of sodium carbonate varies greatly, the rate of calcium ion substitution is faster, and the change is noticeable when the mass fraction of sodium carbonate is less than 11.0%. As the mass fraction of sodium carbonate continues to rise, the replacement rate tends to slow; the replacement difference between calcium and sodium ions first increases and then reaches equilibrium. When the mass fraction of sodium carbonate is 17.0%, the replacement difference of calcium ions comes to the maximum value of 3.1%, and the sedation effect is good.
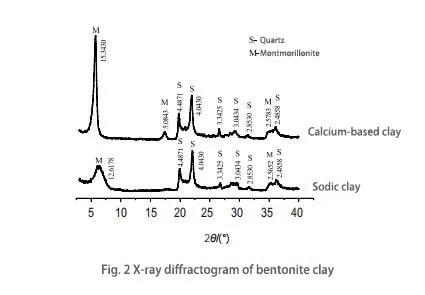
As can be seen from Figure 2, the crystal layer spacing d001 of the bentonite raw ore is 1.53430 nm, which is equivalent to the sum of the thickness of the montmorillonite structural layer (0.96 nm) and the second adsorbed water molecules, which is greater than 1.4 nm, indicating that the specimen is a typical calcium-based bentonite. After sedation, the crystal layer spacing d001 becomes 1.26178 nm, which is because, in the process of sedation, sodium ions enter the interlayer of montmorillonite, replacing the calcium ions with a larger ionic radius, which leads to a decrease in the spacing of the crystal layers and a reduction in the characterization value. The peak bottom angle of the crystal layer spacing d001 becomes more expansive, and the peak decrease becomes gentler, which indicates that the effect of calcium-based bentonite after sedation is noticeable.
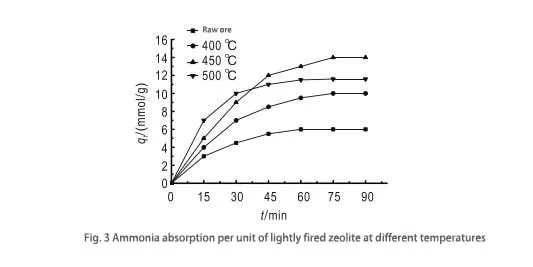
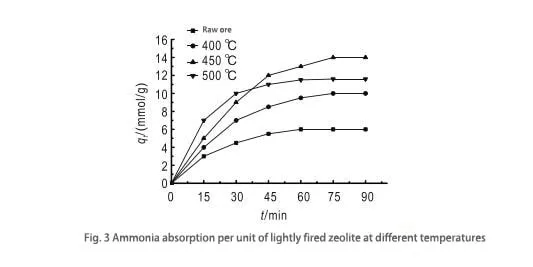
By observing Fig. 3, it can be learned that the best activation effect is achieved at 450°C. This is because the appropriate activation temperature can remove water molecules and residual impurities in zeolite’s molecular structure and form many cavities, thus enhancing its adsorption capacity. After activation, the zeolite undergoes solid fusion and produces a small amount of phase change; while the contact area between the particles increases, the bonding is stronger, and the molecular strength is enhanced, which is favorable for the adsorption process. However, the ammonia adsorption of zeolite activated at 500°C decreases instead. This is because when the temperature is too high, the molecular structure of zeolite will be destroyed, the crystal lattice will collapse, the tiny pores will be closed, the pores will be blocked, and the total area of the solid particles will be reduced, thus affecting the adsorption capacity.
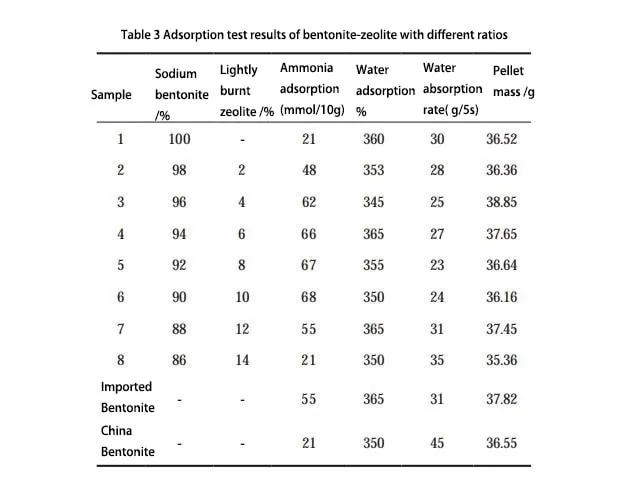
According to the data in Figure 4, when the sodium bentonite and lightly burned zeolite dosage ratio is 94:6, the ammonia adsorption, water adsorption, and water adsorption rates have the best-combined effect. Compared with foreign and domestic high-quality cat litter, the performance of this proportion of the sample is significantly better.
Compared with calcium-based soil, sodium-based bentonite significantly enhances water absorption and expansion times and can be better dispersed in water. The semi-dry sodiated bentonite has improved thermal stability and adhesion. Replacing tetrahedral Si4+ in the montmorillonite structure with Al3+, Fe3+, or A13+, Fe3+ in the octahedron disrupts the electrovalent balance. It, therefore, relies on the metal cations adsorbed in the interlayers to balance the electrovalence. In preparing cat litter, bentonite clay absorbs water and swells when water is added, forming a gel structure. An indeterminate number of bentonite flakes are dispersed from the original crystal structure due to shear and squeezing forces. The basal surface of the lamellae is negatively charged, whereas the Si-O and Al-O bonds of SiO2 and Al2O3 are broken on the cross-sections at the edges, and the lamellae are positively charged on the cross-sections.
During compression, the lamellar bentonite particles are electrostatically adsorbed in three lowest-energy ways, including “face-to-face,” “edge-to-face,” and “edge-to-edge.” Through electrostatic interaction, the bentonite formed a three-dimensional gel structure that resembled a room of cards. Activated zeolites are uniformly dispersed in this bentonite gel structure, which is stable and robust.
Sodium bentonite not only plays the role of water and ammonia absorption in cat litter pellets but also acts as a binder to encapsulate the activated zeolite particles, thus enhancing the strength and adsorption effect of the cat litter particles. When the ratio of bentonite and zeolite is 94:6, the new composite cat litter has a high water-absorbing impact and absorbs ammonia well, so it has a good market prospect.